EB GLASS p.87
reduction to the ferrous state. Lead gives a pale yellow
colour. Silver oxide, mixed as a paint and spread on the surface
of a piece of glass and heated, gives a permanent yellow
stain. Finely divided vegetable charcoal added to a soda-lime
glass gives a yellow colour. It has been suggested that the
colour is due to sulphur, but the effect can be produced with a
glass mixture containing no sulphur, free or combined, and by
increasing the proportion of charcoal the intensity of the colour
can be increased until it reaches black opacity. Selenites and
selenates give a pale pink or pinkish yellow. Tellurium appears
to give a pale pink tint. Nickel with a potash-lead glass gives a
violet colour, and a brown colour with a soda-lime glass. Copper
gives a peacock-blue which becomes green if the proportion of the
copper oxide is increased. If oxide of copper is added to a glass
mixture containing a strong reducing agent, a glass is produced
which when first taken from the crucible is colourless but on
being reheated develops a deep crimson - ruby colour. A similar
glass. if its cooling is greatly retarded, produces throughout
its substance minute crystals of metallic copper, and closely
resembles the mineral called aventurine. There is also an
intermediate stage in which the glass has a rusty red colour by
reflected light, and a purple- blue colour by transmitted light.
Glass containing gold behaves in almost precisely the same way,
but the ruby glass is less crimson than copper ruby glass. J. E.
C. Maxwell Garnett, who has studied the optical properties of
these glasses, has suggested that the changes in colour
correspond with changes effected in the structure of the metals
as they pass gradually from solution in the glass to a state of
crystallization.
Owing to impurities contained in the materials from which
glasses are made, accidental coloration or discoloration is often
produced. For this reason chemical agents are added to glass
mixtures to remove or neutralize accidental colour. Ferrous oxide
is the usual cause of discoloration. By converting ferrous into
ferric oxide the green tint is changed to yellow, which is less
noticeable. Oxidation may be effected by the addition to the
glass mixture of a substance which gives up oxygen at a high
temperature, such as manganese dioxide or arsenic trioxide. With
the same object, red lead and saltpetre are used in the mixture
for potash-lead glass. Manganese dioxide not only acts as a
source of oxygen, but develops a pink tint in the glass, which is
complementary to and neutralizes the green colour due to ferrous
oxide.
Glass is a bad conductor of heat. When boiling water is poured
into a glass vessel, the vessel frequently breaks, on account of
the unequal expansion of the inner and outer layers. If in the
process of glass manufacture a glass vessel is suddenly cooled,
the constituent particles are unable to arrange themselves and
the vessel remains in a state of extreme tension. The surface of
the vessel may be hard, but the vessel is liable to fracture on
receiving a trifling shock. M. de la Bastie's process of "toughening
glass consisted in dipping glass, raised to a temperature
slightly below the melting-point, into molten tallow. The surface
of the glass was hardened, but the inner layers remained in
unstable equilibrium. Directly the crust was pierced the whole
mass was shattered into minute fragments. In all branches of
glass manufacture. the process of "annealing," i.e.ˇ
cooling the manufactured objects sufficiently slowly to allow the
constituent particles to settle into a condition of equilibrium,
is of vital importance. The desired result is obtained either by
moving the manufactured goods gradually away from a constant
source of heat, or by placing them in a heated kiln and allowing
the heat, gradually to die out.
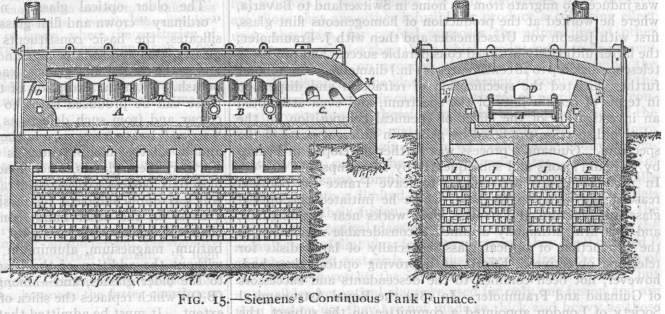 The furnaces (FIG. 15) employed for melting glass are usually
heated with gas on the "Siemens", or some similar system of
regenerative heating. In the United States natural gas is used
wherever it is available. In some English works coal is still
employed for direct heating with various forms of mechanical
stokers. Crude petroleum and a thin tar, resulting from the
process of enriching water-gas with petroleum, have been used
both with compressed air and with steam with considerable success.
Electrical furnaces have not as yet been employed for ordinary
glass-making on a commercial scale, but the electrical plants
which have been erected for melting and moulding quartz suggest
the possibility of electric heating being employed for the
manufacture of glass. Many forms of apparatus have been tried for
ascertaining the temperature of glass furnaces. It is usually
essential that some parts of the apparatus shall be made to
acquire a temperature identical with the temperature to be
measured. Owing to the physical changes produced in the material
exposed prolonged, observations of temperature are impossible. In
the Féry radiation pyrometer this difficulty is obviated, as the.
instrument may be placed at a considerable distance from the
furnace. The radiation passing out front an opening in. the
furnace falls upon a concave mirror in a telescope and is focused
upon a thermoelectric couple. The hotter the furnace the greater
is the rise of temperature of the couple. The electromotive force
thus generated is measured by a galvanometer, the scale of which
is divided and figured so that the temperature may be directly
read. (See THERMOMETRY.)
The furnaces (FIG. 15) employed for melting glass are usually
heated with gas on the "Siemens", or some similar system of
regenerative heating. In the United States natural gas is used
wherever it is available. In some English works coal is still
employed for direct heating with various forms of mechanical
stokers. Crude petroleum and a thin tar, resulting from the
process of enriching water-gas with petroleum, have been used
both with compressed air and with steam with considerable success.
Electrical furnaces have not as yet been employed for ordinary
glass-making on a commercial scale, but the electrical plants
which have been erected for melting and moulding quartz suggest
the possibility of electric heating being employed for the
manufacture of glass. Many forms of apparatus have been tried for
ascertaining the temperature of glass furnaces. It is usually
essential that some parts of the apparatus shall be made to
acquire a temperature identical with the temperature to be
measured. Owing to the physical changes produced in the material
exposed prolonged, observations of temperature are impossible. In
the Féry radiation pyrometer this difficulty is obviated, as the.
instrument may be placed at a considerable distance from the
furnace. The radiation passing out front an opening in. the
furnace falls upon a concave mirror in a telescope and is focused
upon a thermoelectric couple. The hotter the furnace the greater
is the rise of temperature of the couple. The electromotive force
thus generated is measured by a galvanometer, the scale of which
is divided and figured so that the temperature may be directly
read. (See THERMOMETRY.)
In dealing with the manufacture of glass it is convenient to
group the various branches in the following manner:
| |
Manufactured Glass. |
|
| |
I. Optical Glass |
|
| |
II. Blown Glass |
|
| A. Table glass |
B. Tube.
|
C. Sheet |
D. Bottles. |
| |
Special glasses
for thermo-
meters, and
other special
glasses.
|
and crown glass.
|
|
| |
III. Mechanically Pressed
Glass |
|
| A. Plate and rolled plate glass |
B. Pressed table glass |
|
| |
|
|
|
I. OPTICAL GLASS. As regards both mode of production and
essential properties optical glass differs widely from all other
varieties. These differences arise primarily from the fact that
glass for optical uses is required in comparatively large and
thick pieces, while for most other purposes glass is used in the
form of comparatively thin sheets; when, therefore, as a
consequence
 The furnaces (FIG. 15) employed for melting glass are usually
heated with gas on the "Siemens", or some similar system of
regenerative heating. In the United States natural gas is used
wherever it is available. In some English works coal is still
employed for direct heating with various forms of mechanical
stokers. Crude petroleum and a thin tar, resulting from the
process of enriching water-gas with petroleum, have been used
both with compressed air and with steam with considerable success.
Electrical furnaces have not as yet been employed for ordinary
glass-making on a commercial scale, but the electrical plants
which have been erected for melting and moulding quartz suggest
the possibility of electric heating being employed for the
manufacture of glass. Many forms of apparatus have been tried for
ascertaining the temperature of glass furnaces. It is usually
essential that some parts of the apparatus shall be made to
acquire a temperature identical with the temperature to be
measured. Owing to the physical changes produced in the material
exposed prolonged, observations of temperature are impossible. In
the Féry radiation pyrometer this difficulty is obviated, as the.
instrument may be placed at a considerable distance from the
furnace. The radiation passing out front an opening in. the
furnace falls upon a concave mirror in a telescope and is focused
upon a thermoelectric couple. The hotter the furnace the greater
is the rise of temperature of the couple. The electromotive force
thus generated is measured by a galvanometer, the scale of which
is divided and figured so that the temperature may be directly
read. (See THERMOMETRY.)
The furnaces (FIG. 15) employed for melting glass are usually
heated with gas on the "Siemens", or some similar system of
regenerative heating. In the United States natural gas is used
wherever it is available. In some English works coal is still
employed for direct heating with various forms of mechanical
stokers. Crude petroleum and a thin tar, resulting from the
process of enriching water-gas with petroleum, have been used
both with compressed air and with steam with considerable success.
Electrical furnaces have not as yet been employed for ordinary
glass-making on a commercial scale, but the electrical plants
which have been erected for melting and moulding quartz suggest
the possibility of electric heating being employed for the
manufacture of glass. Many forms of apparatus have been tried for
ascertaining the temperature of glass furnaces. It is usually
essential that some parts of the apparatus shall be made to
acquire a temperature identical with the temperature to be
measured. Owing to the physical changes produced in the material
exposed prolonged, observations of temperature are impossible. In
the Féry radiation pyrometer this difficulty is obviated, as the.
instrument may be placed at a considerable distance from the
furnace. The radiation passing out front an opening in. the
furnace falls upon a concave mirror in a telescope and is focused
upon a thermoelectric couple. The hotter the furnace the greater
is the rise of temperature of the couple. The electromotive force
thus generated is measured by a galvanometer, the scale of which
is divided and figured so that the temperature may be directly
read. (See THERMOMETRY.)